Potassium Metal Reacts With Silver Chloride
Aluminum metal reacts with silver sulfate solution to form aluminum sulfate solution and silver metal. - Magnesium reacts only with hot water to form magnesium hydroxide.

Balance Agno3 Kcl Kno3 Agcl Silver Nitrate And Potassium Chloride Youtube
- Calcium reacts less violently with water to form calcium hydroxide.

. When bromide reacts with metal halides like copper II bromide in its aqueous state it forms complexes. Potassium bromide is a strong electrolyte as it can. And water and does not catch fire.
2 Al 3 Ag SO 4 aq Al2SO 43 aq 3 Ag 8. Add concentrated sulfuric acid to solid metal chloride compound. Metal chlorate --- metal chloride and water.
The excretion of 14C-labeled cyanide in rats exposed to chronic intake of potassium cyanide was studied in rats exposed to daily intake of labeled potassium cyanide in the diet for 6 weeks. Introducing heat to K 2 Cr 2 O 7 decomposes it into potassium chromate K 2 CrO 4 and produces O 2 gas. Together with hydrogen they constitute group 1 which lies in the s-block of the periodic tableAll alkali metals have their outermost electron in an s-orbital.
Ammonium nitrate --- water and dinitrogen oxide laughing gas There are three ways to break down chemicals into simpler substances. Silver chloride --- silver and chlorine. When the solution of K 2 Cr 2 O 7 reacts with an alkali ionic salt a yellow solution is obtained because of the potassium.
It is an ionic compound with two potassium ions K and the negatively charged dichromate ion Cr2O7- in which two hexavalent chromium atoms with oxidation state 6 are each attached to three. State three uses of hydrochloric acid. Then Silver chloride a white precipitate is formed when aqueous chloride solution is added to silver nitrate AgNO 3 solution.
Depending on the design and chemical compounds used lithium cells can produce voltages from 15 V comparable to a zinccarbon. HNO 3 reacts to form hascent chlorine which reacts with Gold or Platinum to form their respective soluble chlorides. Calcium hydroxide hydrochloric acid calcium chloride water.
Lithium batteries are primary batteries that have metallic lithium as an anodeThese types of batteries are also referred to as lithium-metal batteries. Silver ions can dissolve halide anions out. One way in which this can be done is by employing potassium chromate as indicator.
Potassium is a soft silvery-white metal member of the alkali group of the periodic chart. When one chemical reacts to a stimulus by breaking into two simpler elements. Mercuric oxide --- mercury metal and oxygen.
I It is used in the manufacture of silver chloride which is used widely in photography. They stand apart from other batteries in their high charge density and high cost per unit. - Metals like aluminium iron and zinc react only with steam to form.
This shared electron configuration results in their having very similar characteristic. Non-metal non-metal C O2 CO2 carbon dioxide N2 3 Cl2 2 NCl3 nitrogen trichloride 2. The metal oxides and hydrogen.
Iron metal reacts with bromine gas to form iron III. Potassium chromate reacts with the first slight excess silver ion to form a red precipitate of silver chromate. Presence of solid metal chloride compounds can be tested from this.
4K 2 Cr 2 O 7 4K 2 CrO 4 2Cr 2 O 3 3O 2. For the chemical reaction between an acid and a metal hydroxide base the products are a salt and water. A metal non-metal 2 Na Cl2 2 NaCl sodium chloride Fe S FeS ironII sulfide b.
Chemical Properties of Potassium Dichromate. The alkali metals consist of the chemical elements lithium Li sodium Na potassium K rubidium Rb caesium Cs and francium Fr. Potassium bromide has ionic bonding between its two elements potassium and bromine.
CH 4 2 O2 g CO 2 g 2 H2O g 9. The chemistry of potassium is. Potassium is silvery when first cut but it oxidizes rapidly in air and tarnishes within minutes so it is generally stored under oil or grease.
It is light enough to float into water with which it reacts instantly to release hydrogen which burns with a lilac flame. Methane gas CH 4 reacts with oxygen gas to form carbon dioxide gas and water vapor. Metal oxide non-metal oxide K2O SO3 K2SO4 potassium sulfur potassium oxide trioxide sulfate CaO CO2 CaCO3 calcium carbon calcium oxide dioxide carbonate c.
Bromine has a -1 charge and potassium has a 1 charge. If we are given the following word equation. Water will also be a product when a metal hydroxide reacts with an acid.
- Metals like lead copper silver and gold do not. Urinary excretion was the main route of elimination of cyanide carbon in these rats accounting for 83 of the total excreted radioactivity in 12 hr and 89 of the total excreted radioactivity in 24 hr. AgNO 3aq Cl-aq AgCl s NO 3-aq Silver chloride is insoluble in water.
Metal hydroxide acid salt water. The presence of the first slight excess of silver ion ie the end point can be marked by the appearance of a coloured precipitate. Ii It is used in the manufacture of dyes drugs and.
The chemical formula for potassium dichromate is K 2 Cr 2 O 7 and the molar mass is calculated to be 294185 gmol. Potassium Dichromate Formula and Molecular Structure.
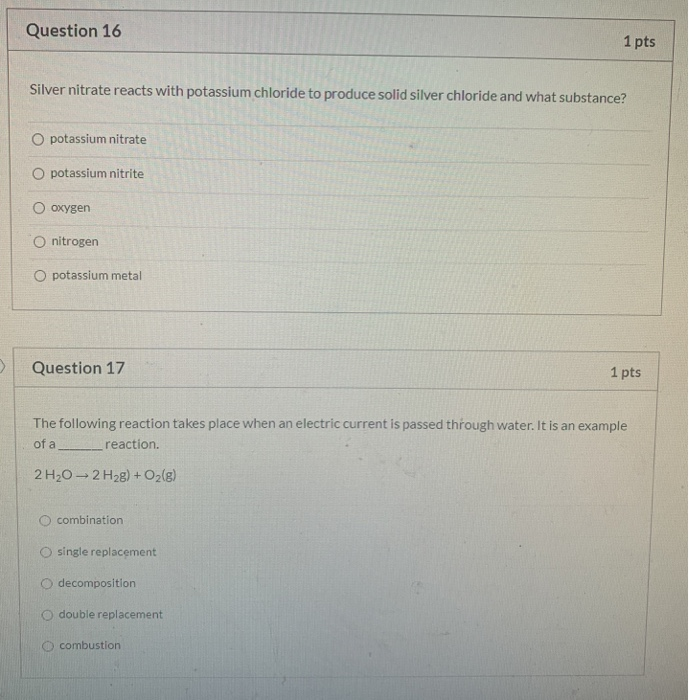
Solved Question 16 1 Pts Silver Nitrate Reacts With Chegg Com

Solved Predicting Rxn Products Worksheet For Each Item Chegg Com

Chemical Equations More Writing And Balancing Practice Go Through This Powerpoint For Extra Tips And Extra Practice Ppt Download
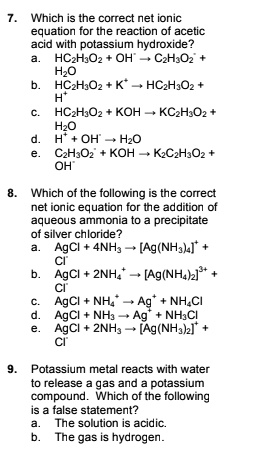
Solved Which Is The Correct Net Ionic Equation For The Reaction Of Acetic Acid With Potassium Hydroxide Hc Hjoz Czhjoz Hzo Hc Hjoz Hc H Oz Hchjoz Koh Kczhjoz Hzo Oh Hzo Czhsoz Koh
Comments
Post a Comment